Native budworm
Helicoverpa punctigera
Other common names
Heliothis

Photo by Andrew Weeks, Cesar Australia
Summary Top
The native budworm, sometimes known as Heliothis, is a common and widespread pest of pulse crops and canola. It occurs in most years and often migrates into agricultural areas from nearby or distant rangelands. Larvae vary widely in colour and can be confused with other pest caterpillars. There are many natural enemies that attack native budworm and mortality is often very high in early stages. Economic thresholds for different crops are available and spraying is recommended once these have been reached and while caterpillars are small (<7 mm). When thresholds are exceeded, native budworms can be controlled with insecticides although timing and coverage are both critical to achieving good control. Windrowing canola or desiccating pulse crops can help to control native budworms.
Occurrence Top
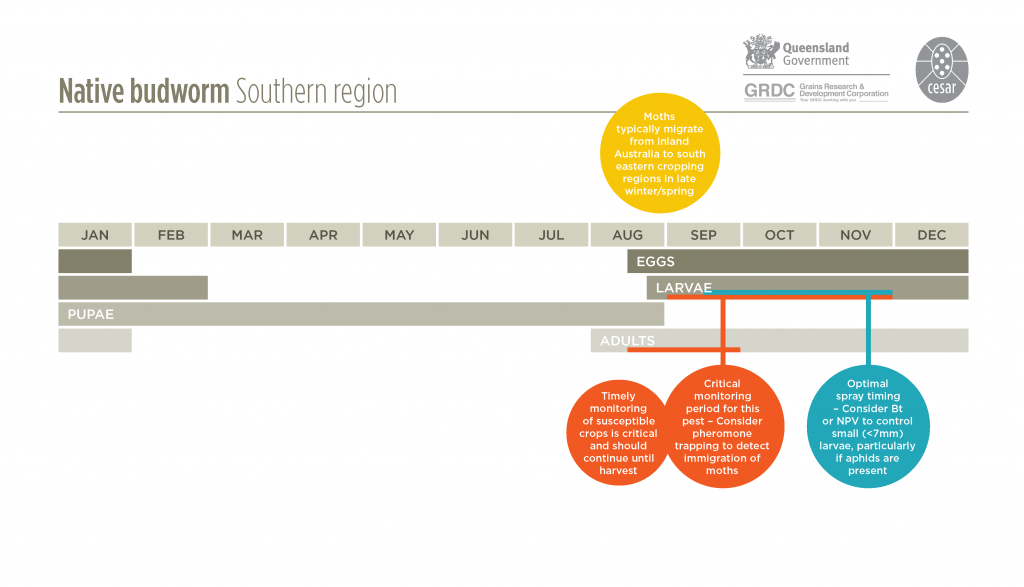
A native species that is common and widely distributed throughout Australia. Native budworm breeds over winter in the arid inland regions of Queensland, South Australia, Western Australia and New South Wales on desert plants before migrating into southern agricultural areas in late winter or spring. They can migrate as far south as Tasmania.
A native species that is common and widely distributed throughout Australia.
Description Top
Native budworm eggs can be found singly on the growing tips and buds of plants. They are small (about 0.5 mm in diameter) but quite visible to the naked eye after close inspection of the plant. They are white when first laid but change colour from yellow through to brown, as they get closer to hatching.
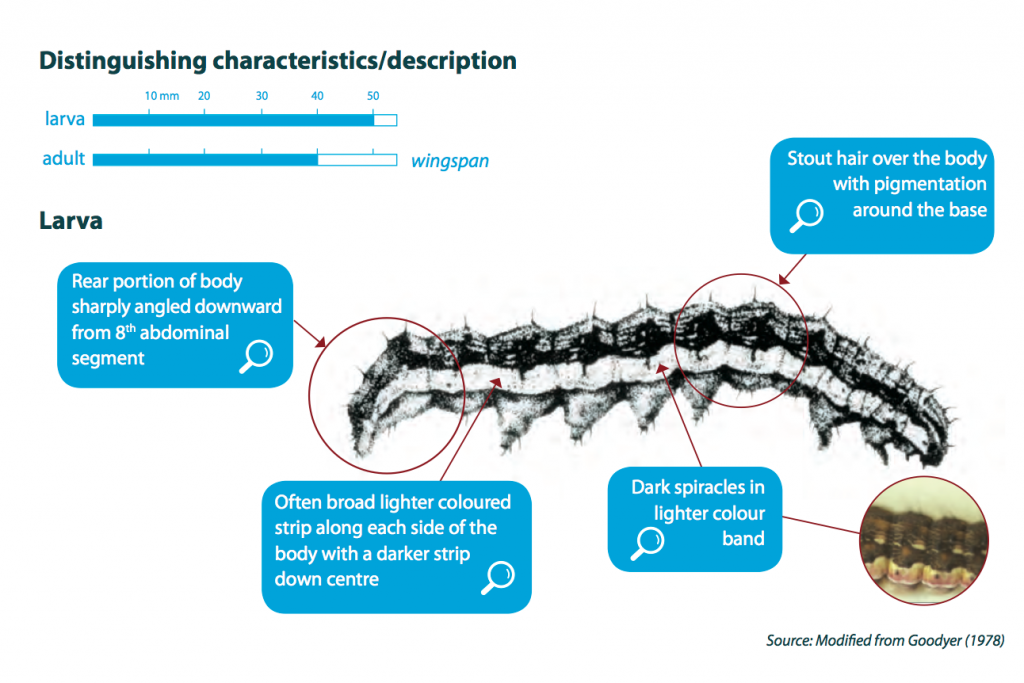
Newly hatched caterpillars (larvae) are very small (approximately 1.5 mm), light in colour with dark brown heads. They can easily be missed when inspecting a crop. They will grow through six or seven stages or instars until reaching maturity (up to 40 mm long).
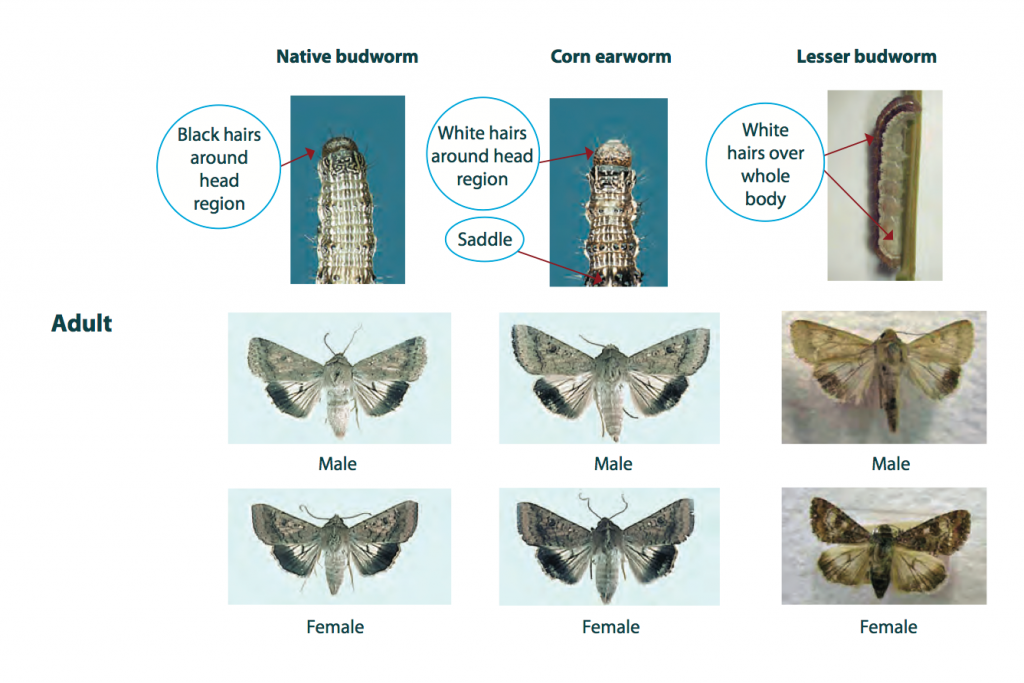
Larvae vary substantially in colour that includes shades of brown, green and orange. Regardless of colour, they usually have darkish stripes along the body and bumpy skin with sparse, stiff, stout hairs. One distinguishing feature of Helicoverpa larvae is the sharp downward angling at the rear of the body. However, in contrast to cotton bollworm (aka corn earworm) and lesser budworm, larvae of native budworm have black hairs around the head and along the body.
Adult moths are approximately 15-18 mm long and buff, light brown to red-brown in colour, with numerous dark spots and blotches. The hind wings of the adult moth are pale with a dark band along the lower edge and span 30-35 mm.


Lifecycle Top
Native budworms breed during winter on flowering plants in inland Australia when there is sufficient rainfall for broadleaf vegetation to flourish. When this inland vegetation dies off, typically in late winter/spring, the larvae pupate and moths emerge in readiness to migrate, often to the southern and eastern agricultural regions (or western regions of Western Australia). Migratory moths reach the cropping areas by flying vertically up into the warm northerly or northwesterly winds that precede cold fronts in the winter/ spring period. Hence, the moths that are frequently encountered in crops during early spring may well have had their origins from somewhere in the inland of Australia.
Moths typically lay up to 1000 white spherical eggs singularly near the top of growth points of the plant. Eggs darken as they mature and larvae hatch in 7-14 days. Native budworm larvae develop through six growth stages, becoming fully-grown in 2-3 weeks in summer and 4-6 weeks in spring. They will generally have four to five generations per season. Larval activity increases in warmer conditions, and ceases when temperatures fall below about 12°C.
Behaviour Top
Early instar larvae, less than 7mm, generally feed on leaves, or graze on the surface of pods. Exceptions can be following periods of unseasonably warm when even very small larvae burrow into young pods, making them difficult to monitor.
A notable feature of this pest is its capacity to migrate at high altitudes over large distances (100-1000 km) at night. The moths fly from areas where conditions do not favour another generation to where there are abundant food plants for further breeding.
Moths live for two to four weeks; they rest during the day and become active after sunset, feeding on nectar from flowers and laying eggs on many types of herbaceous plants (weeds and crops). They fly rapidly from plant to plant throughout the night in a darting motion, feeding and laying eggs; this behaviour is often used to recognise budworm moths. They are also capable of flying from paddock to paddock and of course, from one region to another.
Similar to Top
Budworm caterpillars and moths resemble those of the lesser budworm and the corn earworm (or cotton bollworm). They may also be confused with armyworms and cutworms that have smooth bodies without the stout hairs.
Crops attacked Top
Native budworm prefer broadleaf plants and are rarely found on grass or cereal crops. They attack pulse crops such as field peas, faba beans, lentils, chickpeas, lupins and soybeans, as well as canola, lucerne, sunflower, safflower, annual medic and clovers.
Damage Top
Budworms are at their most damaging when they feed on the fruiting parts and seeds of plants. During the formation and development of pods, field pea, chickpea, lentil and faba bean crops are very susceptible to all sizes of native budworm caterpillars. Small caterpillars can enter emerging pods and damage developing seed while larger caterpillars may devour the entire pod contents.
Narrow-leafed lupin and canola crops will not be damaged by native budworm until they are close to maturity and leaf fall commences.
Holes or chewing damage may be seen on pods and/or seed heads. Grubs may be seen or may remain embedded in the pod. Losses attributed to budworms come from direct weight loss through seeds being wholly or partly eaten. Grain quality may also be downgraded through unacceptable levels of chewed grain.
Caterpillars eat increasing quantities of seed and plant material as they grow. The last two growth stages (5th & 6th instar) account for over 90% of their total grain consumption.

Monitor Top
The use of pheromone traps (which attract male moths) provides an early warning of moth arrival and abundance, following their migration from inland regions. These should be set up in late winter or early spring. Observing the activity of moths in the crop and the presence of eggs may also be indicative of future larval activity. However, egg and early larval mortality of native budworm through natural courses can be very high. In Queensland chickpeas, this has been estimated to be up to 70%. Eggs and very small larvae can be dislodged and will die after heavy rain or wind.
Either a sweep net (eg. peas, lentils) or beat sheet (eg. chickpeas, lupins, beans) should be used to monitor larval activity in crops. Monitor field pea, faba bean, lentil and chickpea crops from budding and flowering development through to maturity for larval activity. Monitor narrow leaf lupin and canola crops at pod development to maturity. Native budworm infestations are most problematic in spring and early summer, and may be greater when aphid infestations are heavy as the moths are attracted to the sugary honeydew produced by aphids.
Sweep netting is quick and easy method to sample most short crops such as lentils, peas or vetch (or cereals). They may be less efficient in tall dense crops of faba beans, lupins and chickpeas, although still useful to give relative counts. Monitor crops for activity by taking a minimum of 5 sets of 10 sweeps and calculating the average number of larvae per 10 sweeps. Each sweep should consist of moving the net through the crop in an arc of approximately 180°. See the following short You Tube video on sweep netting:https://www.youtube.com/watch?v=fwesXzQ1pCc
A beat sheet is a good alternative monitoring technique for taller or more rigid crops, especially wide row chickpeas and faba beans. A standard beat sheet (plastic or canvass) is about 1.3 metres wide and 1.5 metres long, with a heavy dowel to weigh it down. One edge should be placed at the base of a row and the sheet spread out across the inter row space. If rows are narrow then it can be draped over the adjacent row. Using a 1 metre long rod, vigorously shake the row 10 times over the sheet to dislodge and catch larvae. The following You Tube video demonstrates the use of a beat sheet in chickpeas: https://www.youtube.com/watch?v=_Z457Cc1fig
During monitoring, record numbers of larvae according to size; very small (< 3 mm), small (4-7 mm), medium (8-23 mm) and large (>24 mm). Numbers of larvae used to calculate economic thresholds exclude very small larvae. Large larvae cause 90% of the damage.

Economic thresholds Top
Comprehensive and dynamic economic thresholds have been developed for native budworm in Western Australia (Mangano et al. 2006), which should also apply to south-eastern Australia. Below is an example, assuming cost of control at $10/ha. Control is warranted if the cost of control is less than the value of the yield loss predicted.
Estimates using other grain price and control costs can be estimated by applying the formula: ET = (C x 1000) / (K x P).
| K – grain loss kg/larva/ha | P – grain price $/tonne | C – cost of control $/ha | ET – larvae per 10 sweeps* | |
| Field peas | 50 | 400 | 10 | 0.5 |
| Lentils | 60 | 680 | 10 | 0.3 |
| Faba bean | 90 | 500 | 10 | 0.2 |
| Chickpeas – desi | 30 | 600 | 10 | 0.6 |
| Canola | 6 | 450 | 10 | 4.0 |
| Lupins | 7 | 420 | 10 | 4.0 |
* Numbers exclude very small larvae.
Applying these thresholds to chickpeas, as an example, provides a means to assess the value of the consumed grain.
| Value of yield loss ($/ha) | |||||
| Chickpea price ($/t) | 1 larva/10 sweeps | 2 larvae/10 sweeps | 3 larvae/10 sweeps | 4 larvae/10 sweeps | 5 larvae/10 sweeps |
| 200 | 6 | 12 | 18 | 24 | 30 |
| 300 | 9 | 18 | 27 | 36 | 45 |
| 400 | 12 | 24 | 36 | 48 | 60 |
| 500 | 15 | 30 | 45 | 60 | 75 |
| 600 | 18 | 36 | 54 | 72 | 90 |
Lupins and canola will have much higher thresholds than other pulses. For other crops, the threshold is below 1 larva per 10 sweeps.
Apart from yield loss, premium downgrades are also a possibility because of budworm damage. The large seeded pulses, especially faba beans, are particularly susceptible to being downgraded through receival standards at the silo because of nibbled or defective grain. Partial seed damage is more likely than in smaller seeded pulses.Applying these thresholds to chickpeas, as an example, provides a means to assess the value of the consumed grain.
Management options Top
Biological
A key component to any IPM is to maximise the number of beneficial organisms and incorporate management strategies that reduce the need for pesticides. Correct identification and monitoring is the key when checking for build up or decline in beneficials. There are many natural enemies that attack native budworm. The egg stage is susceptible to the parasite Trichogramma ivalae, a minute wasp that has been recorded in up to 60% of eggs along with egg predators such as ladybird beetles, lacewings and spiders. Beneficials attacking larvae include shield bugs, damsel bugs, assassin bugs, tachinid flies (their larvae prey on caterpillars), orange caterpillar parasite, two-toned caterpillar parasite, orchid dupe, lacewings and spiders. Naturally occurring fungal diseases and viruses also play an important role in some seasons.
Cultural
Windrowing canola or desiccating pulse crops such as field peas may be an option to advance the drying of crops when small-medium size larvae are present near crop maturity.
Chemical
There are several insecticides registered for the control of native budworm. Timing and coverage are both critical to achieving good control. Try to target small larvae up to 7 mm in length and apply insecticides before larvae move into flowering pods. IPM options include the use of Bt (Bacillus thuringiensis) and nuclear polyhedrosis virus (NPV) based biological insecticides. Small larvae are generally easier to control because they are more susceptible to insecticides, and leaf feeding makes them susceptible to ingestion of active residues on the plant surface. Larvae entrenched in buds and pods will be more difficult to control and chemical residual will be important in contacting them.
The crop should be re-inspected 2 to 4 days after spraying to ensure enough caterpillars have been killed to prevent future damage and economic loss. In years of very high moth activity and extended egg lays, a second spray may be required.
In choosing a registered product, be aware of the withholding period for harvest or windrowing/ swathing which is the same as harvest. Residue testing is routinely conducted on grain destined for export and domestic stock feed markets.
Timing and coverage are both critical to achieving good insecticidal control of native budworms
Acknowledgements Top
This article was compiled by Garry McDonald (cesar).
References/Further Reading Top
Anon. 2005. Understanding Helicoverpa ecology and biology in Southern Queensland: know the enemy to manage it better. Agdex No. 612. Queensland Department of Primary Industries and Fisheries.
Baker GH, Tann CR and Fitt GP. 2011. A tale of two trapping methods: Helicoverpa spp. methods: (Lepidoptera, Noctuidae) in pheromone and light traps in Australian cotton production systems. Bulletin of Entomological Research 101: 9-23.
Bailey PT. 2007. Pests of field crops and pastures: Identification and Control. CSIRO Publishing, Melbourne, Australia.
Bellati J, Mangano P, Umina P and Henry K. 2010. I SPY Insects of Southern Australian Broadacre Farming Systems Identification Manual and Education Resource. Department of Primary Industries and Resources South Australia (PIRSA) and the Department of Agriculture and Food Western Australia (DAFWA).
Gu H, Fitt GP and Baker GH. 2007. Invertebrate pests of canola and their management in Australia: a review. Australian Journal of Entomology 46: 231-243.
Mangano P, Michael P and Hardie D. 2006. Management of native budworm in pulse and canola crops in the south-west of Western Australia. Farmnote: 184. Department of Agriculture and Food Western Australia.
McDonald G. 1995. Native budworm. AG0417. Victorian Department of Primary Industries.
Murray DAH, Clarke MB and Ronning. 2013. Estimating invertebrate pest losses in six major Australian grain crops. Australian Journal of Entomology 52: 227-241.
Robin G. 2012. Insecticide Resistance in Helicoverpa spp. Inside Cotton.
| Date | Version | Author(s) | Reviewed by |
|---|---|---|---|
| February 2015 | 1.0 | Garry McDonald (cesar) | Greg Baker (SARDI) |
What are PestNotes?
PestNotes are information sheets developed through a collaboration between Cesar Australia and the South Australian Research and Development Institute (SARDI). Copyright: © All material published in PestNotes is copyright protected by Cesar Australia and SARDI and may not be reproduced in any form without written permission from both agencies.
Disclaimer
The material provided in PestNotes is based on the best available information at the time of publishing. No person should act on the basis of the contents of this publication without first obtaining independent, professional advice. PestNotes may identify products by proprietary or trade names to help readers identify particular products. We do not endorse or recommend the products of any manufacturer referred to. Other products may perform as well as or better than those specifically referred to. Cesar Australia and PIRSA will not be liable for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information in this publication. Any research with unregistered pesticides or products referred to in PestNotes does not constitute a recommendation for that particular use.

