Australian plague locust
Chortoicetes terminifera

Photo by Andrew Weeks, Cesar Australia
Summary Top
Native to Australia, occurring primarily in inland breeding areas including parts of Queensland, New South Wales, South Australia and Western Australia. Adults are identifiable by a black spot on the tip of the hind wing. Outbreaks occur in some seasons when dense swarms of adult locusts can invade pastoral and cropping areas of southern Australia. Up-to-date advice can be obtained from the Australian Plague Locust Commission, and South Australian, Victorian or New South Wales State departments of primary industries.
Landholders are responsible for the control of locusts on their land.
Occurrence Top
The Australian plague locust is native to Australia and inhabits a wide expanse of inland Australia including parts of Queensland, New South Wales, South Australia, Western Australia and the Northern Territory. Outbreaks occur in some seasons following favourable conditions in inland breeding areas that result in extensive population build-up over sequential generations. High density swarms may develop and migrate long distances on suitable wind systems into cropping regions of New South Wales, Victoria and South Australia.
Description Top
Adults of the Australian Plague locust are identified by a characteristic black spot on the tip of the hind-wing, red shanks on the hind-legs and a distinct ‘X’ shaped mark behind the head. They are generally mottled brown in colour but can be grey or green. Adult males are 25-30 mm in length while females are somewhat larger at 30-45 mm. Nymphs are similar to adults but are wingless, and usually brown or green with dark legs. After hatching, nymphs develop through 5 growth stages (instars), with wing buds becoming more prominent at each stage. A full guide on the identification of the Australian plague locusts and other related grasshoppers can be found on this link in the Australian Plague Locust Commission (APLC) website.


Lifecycle Top
Adults with adequate green feed can become sexually mature about 2 weeks after developing wings. Females select suitable sites for egg-laying, usually in areas of hard bare ground (such as clay-pans, tracks and road verges) or heavier soils, but occasionally lighter soils. Before laying, females may test-drill with their abdomen to assess soil condition. Eggs are laid in pods of 30-50 eggs at about 3-10 cm depth, and females usually lay 2-3 pods. Egg-laying activity is often aggregated, forming dense ‘egg beds’.
Egg development is dependent on soil and moisture conditions, and may be delayed (via quiescence) if conditions are too cold or dry. Eggs laid in summer may develop directly and hatch in 2-4 weeks. In southern areas, the majority of eggs laid during autumn enter winter diapause, resuming development in late winter and hatching in spring.
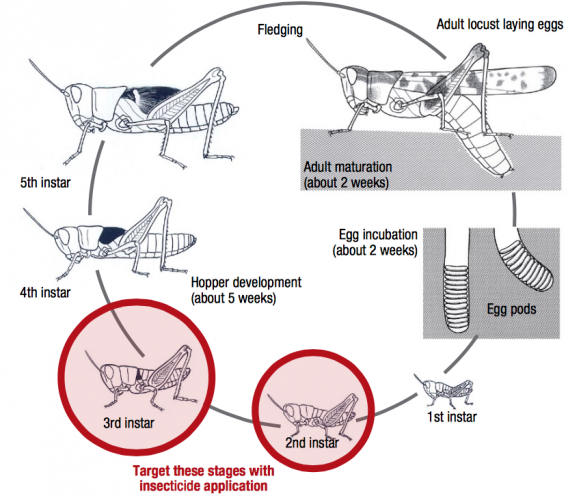
After hatching, nymphs undergo five growth stages before fledging into winged adults, completing development in 4-6 weeks. Adults require green feed for growth, to develop eggs in their ovaries, and accumulate fat reserves as fuel for migration. There are often three generations per year, approximately corresponding to spring, autumn, and summer generations, but may vary from to two to four depending on region and seasonal conditions.
Behaviour Top
Adult locusts migrate into agricultural areas from inland pastoral regions when favourable weather conditions enable population expansion and migration. Nymphs at the 2nd and 3rd instar stages form dense aggregations, called ‘bands’ that march and feed on green vegetation. Bands may match up to 500 metres per day in any direction. Adult locusts can form high density swarms that migrate long distances to areas where green feed is available. These swarms can devastate crops in spring.
Chemical control should target dense bands of wingless hoppers at the 2nd and 3rd instar stage.
Similar to Top
Small plague grasshopper and wingless grasshopper
Crops attacked Top
All pastures and field crops containing green plant material are at risk. Perennial pastures such as lucerne, long season annual pastures and newly sown pastures are at greatest risk of attack later in the season, since they tend to remain green longer.
Damage Top
Swarms of adult locusts may fly in to crops or pastures and feed, causing severe damage. In cereals, it is the spring generation that affects crops. Less commonly, swarms may also fly into cropping areas in autumn and damage newly emerging crops.
Adults and nymphs can remove all green plant material in leaves, stems and grain heads. Maturing cereals are vulnerable to lopping of grain heads as locusts chew through the last remaining green material in the stem below the head. High density swarms are capable of completely devastating a crop overnight. In pastures, it has been estimated that 20 hoppers per m² eat the same as 3–5 dry sheep equivalent (DSE) per hectare. Summer rains and green feed may sometimes allow populations to persist into the following autumn and spring.
Monitor Top
Landholders are responsible for the control of locusts on their own property. Monitor when egg hatching commences in spring. Predicted hatching date guidelines for each region are provided in locust bulletins produced by the APLC. Spring monitoring for hatching of nymphs should focus on areas where adult activity and egg-laying was observed in autumn. The APLC monitors locust populations across all states with the focus on the more arid regions. For warnings of locust outbreaks, information can be found from the ALPC (http://www.agriculture.gov.au/pests-diseases-weeds/locusts/current) or sourced through local state government authorities.
Call the Emergency Plant Pest Hotline on 1800 084 881 to report suspected cases of the Australian Plague Locust.
Economic thresholds Top
Action should take place at first evidence of bands or swarms. Hopper control for pasture protection can be considered economic if hopper densities exceed 20 per m² and adult locust control in pastures is economic if locust densities exceed 10 per m² (APLC/GRDC 2010).
Management options Top
Biological
Parasitoids include the egg parasitoids (Scelio fulgidus, Scelio parvicornis) as well as fly parasitoids (Cryptomorpha flaviscutellaris, Blaesoxipha spp., Trichopsidea oestracea). There are also various predatory beetles that attack Australian plague locusts including beetles in the families Anthicidae, Carabidae and Tenebrionidae. These predators and parasitoids cannot however be relied upon to effectively control large populations of Australian plague locusts.
Cultural
Where significant egg-laying has been detected, deep cultivation can reduce egg survival. Graze pastures or cut for fodder in late winter and early spring before the arrival of locusts.
Chemical
A number of insecticides are registered (or have permits issued) for control of Australian plague locusts, including organophosphates, synthetic pyrethroids, carbamates and Metarhizium based-products. Refer to state-based departments for information. Chemical control should directly target dense bands of wingless hoppers at the 2nd and 3rd instar stage (approx. 6-10mm in length). If control of adults is required, it is recommended to spray in the early morning or late evening when locusts are settled. Bio-pesticides based on the naturally occurring Metarhizium (M. anisopliae) soil fungus specifically targets locusts and grasshoppers, and is primarily used in environmentally sensitive areas.
Acknowledgements Top
This article was compiled by Paul Umina (cesar) and Bill Kimber (SARDI).
References/Further Reading Top
Much of this information was sourced from the APLC website: http://www.agriculture.gov.au/pests-diseases-weeds/locusts/about/australia
APLC and GRDC. 2010. Plague locust control fact sheet. http://www.grdc.com.au/uploads/documents/Plague_Locusts_Factsheets.pdf
Bailey PT. 2007. Pests of field crops and pastures: Identification and Control. CSIRO Publishing, Melbourne, Australia.
Farrow RA (1979). Population Dynamics of the Australian Plague Locust, Chortoicetes Terminifera (Walker), in Central Western New South Wales. I. Reproduction and Migration in Relation to Weather. Australian Journal of Zoology 27: 717 – 745.
Henry K, Bellati J, Umina P and Wurst M. 2008. Crop insects: the Ute Guide, Southern Grain Belt Edition. Government of South Australia PIRSA and GRDC.
Henry K. 2010. The Australian Plague Locust. South Australian Research and Development Institute (SARDI) Factsheet FS 9/10.
Hunter DL, Milner RJ, and Spurgin, PA. 2001. Aerial treatment of the Australian plague locust, Chortoicetes terminifera (Orthoptera: Acrididae) with Metarhizium anisopliae (Deuteromycotina: Hyphomycetes). Bulletin of Entomological Research 91: 93-99.
| Date | Version | Author(s) | Reviewed by |
|---|---|---|---|
| November 2015 | 1.0 | Paul Umina (cesar) and Bill Kimber (SARDI) | Kym Perry (SARDI) and Garry McDonald (cesar) |
What are PestNotes?
PestNotes are information sheets developed through a collaboration between Cesar Australia and the South Australian Research and Development Institute (SARDI). Copyright: © All material published in PestNotes is copyright protected by Cesar Australia and SARDI and may not be reproduced in any form without written permission from both agencies.
Disclaimer
The material provided in PestNotes is based on the best available information at the time of publishing. No person should act on the basis of the contents of this publication without first obtaining independent, professional advice. PestNotes may identify products by proprietary or trade names to help readers identify particular products. We do not endorse or recommend the products of any manufacturer referred to. Other products may perform as well as or better than those specifically referred to. Cesar Australia and PIRSA will not be liable for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information in this publication. Any research with unregistered pesticides or products referred to in PestNotes does not constitute a recommendation for that particular use.

