African black beetle
Heteronychus arator

Photo by Andrew Weeks, Cesar Australia
Summary Top
The African black beetle is an introduced beetle that appears similar to cockchafers but generally occurs earlier in the year and is usually found on or under the soil. Both adults and larvae attack pastures and cereals. The beetles are of considerable economic importance because, while they attack cereals, they also attack a wide range of horticultural crops and long-term pastures. Damage by these beetles and their larvae can be reduced by delaying autumn sowing, or applying insecticide seed treatment.
Occurrence Top
It is native of Africa, now present in Australia and the North Island of New Zealand. A sporadic agricultural pest found in Western Australia, South Australia, Victoria, New South Wales and Queensland, the African black beetle has not been recorded in Tasmania.
Description Top
African black beetle larvae are soil dwelling and are typical white, soft-bodied scarab grubs. They have ‘C’-shaped bodies, six legs and a yellow-brown head capsule with noticeable black jaws. Late stage larvae are 25-30 mm in length. Newly hatched larvae are about 5 mm long. The abdomen towards the rear is generally swollen and darker in colour.
Adults are shiny black, slow moving and have a cylindrical body that is approximately 12-14 mm long. From above, the body sides are almost parallel and the wing covers have lightly indented longitudinal striations.


Lifecycle Top
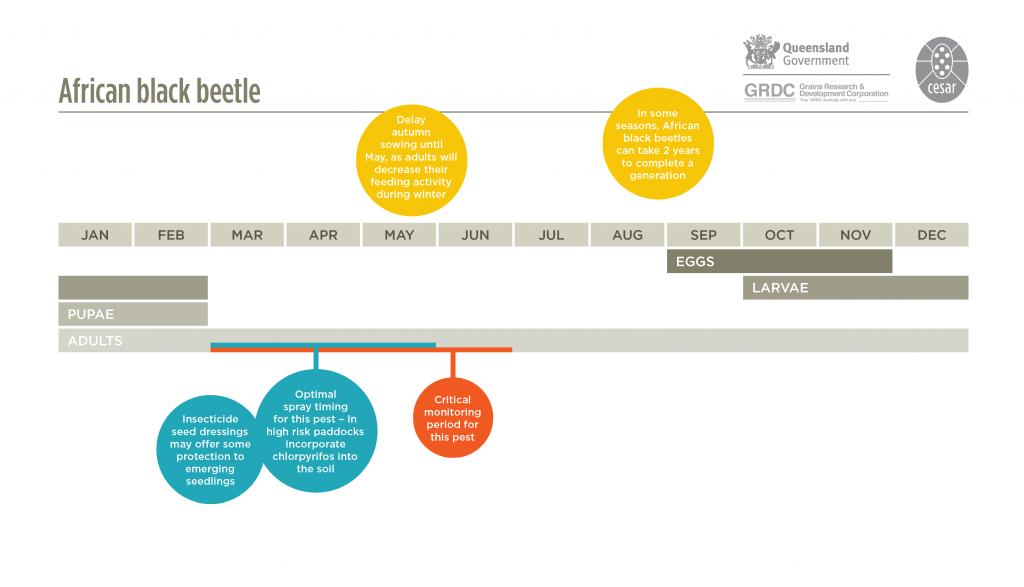
African black beetle has one generation per year, but in some seasons the generation can take two years. Eggs are laid in the soil in spring and hatch in two to five weeks depending on temperature. Larvae hatch and pass through three growth stages before pupating and emerging as adults between late December and early February. The period from eggs to adults is approximately three to four months, while the adult lifespan is about ten months. Adults remain sexually immature until spring when mating and oviposition occurs. Larvae are generally present from late spring to mid-summer but may be found during winter. These are late developers and fail to survive.
Behaviour Top
African black beetles have strong nocturnal flight activity, and disperse during their ‘roaming’ stage leading to crops becoming infested. Flights during summer to autumn can be observed as activity increases around lights on buildings etc. Beetles are attracted to grassy areas or those with heavy mulching.
Adults chew plants at or just beneath ground level and may chew right through the stem or cause ‘ring barking’ on larger plants.
Unlike the cockchafer pests, the African black beetle adult and larvae are both capable of causing severe plant damage.
Similar to Top
Other scarabs and cockchafer larvae, including the yellowheaded cockchafer, redheaded pasture cockchafer and other pasture scarabs.
Adults can be confused with redheaded cockchafer and some dung beetles. Adult redheaded cockchafers can be distinguished by their ovoid body and wing covers with deeply indented striations separated by distinct dots.
Larvae can be distinguished from other yellowheaded scarabs (Sericesthis species) by their anal opening at the tip of the abdomen (raster). African black beetle has a horizontal opening while yellowheaded scarabs have a ‘Y” opening, as illustrated here. Redheaded pasture cockchafers have a distinctive red head capsule.
Crops attacked Top
African black beetles are normally associated with damage to long-term pastures and grasses, turf and some horticulture crops. They also attack several cereal crops including barley, triticale and wheat.
Damage Top
Damage can be caused by adults feeding on the stems of young plants either underground or above the soil surface, often killing growing points so that the central shoots wither and the plants die. Older plants usually survive, but remain weak. Larvae prune or completely sever roots of perennial grasses and in severe cases where larval populations are high, pasture becomes patchy and can be rolled back like a carpet.
Soil-dwelling third instar larvae cause most damage to pastures by cutting of roots below the soil surface.


Monitor Top
Inspect susceptible paddocks prior to sowing. Check crops and newly sown pastures for the presence of adults and damage in autumn-early winter. Adults can be found on or under the soil surface, to a depth of about 15 cm. Monitor crops and pastures in late spring to mid-summer for larval damage. Inspect susceptible paddocks prior to sowing by digging to a depth of 10-20 cm with a spade and counting the number of larvae present. This should be repeated 10-20 times to get an estimate of larval numbers. Four larvae per spade square is roughly equivalent to 100 larvae per m2.
Economic thresholds Top
Beetle densities in excess of 10 per square metre may result in significant crop damage, but control may be warranted with densities of five per square metre or less.
Management options Top
Biological
While there is a commercially available nematode (Heterorhabditis zealandica) for the biological control of African black beetle in turf and other high value crops, these are unlikely to be cost effective in broad acre crops.
Cultural
There are a number of agronomic options that may reduce the intensity of black beetle damage, particularly if they are a continual problem.
Delay autumn sowing until May as adults decrease their feeding activity during winter.
Consider sowing less favourable pastures and crops such as legumes, oats and lucerne. There are also black beetle-resistant endophytes now available in perennial ryegrass, such as AR37 (follow recommendations to avoid stock performance issues).
Increasing seeding rates is a useful option in paddocks where the pest is anticipated to cause damage.
There is some evidence that liming in acid soil environments can reduce the survival of young black field beetle larvae.
Consider removing kikuyu grasses well prior to seeding as this grass is a favoured food plant for black beetle and can sustain high beetle populations. Keep the paddock as bare fallow for as long as is feasible prior to planting. When considering this option assess the susceptibility of the paddock to wind and water erosion.
Delaying autumn sowing and increasing seeding rates, taking advantage of biological control and insecticide seed dressings can reduce damage.
Chemical
Poncho Plus® has recently been registered as a seed protectant against African black beetle and yellow headed pasture cockchafers in pastures. Control is expected for 3-4 weeks after sowing, but will not control heavy populations. Chlorpyrifos is registered in maize however no other foliar insecticide is registered for African black beetle control in broadacre crops. Larvae live underground and are unlikely to be affected by foliar applications of insecticides. Adults may be killed before they lay eggs during spring, however monitoring for the presence of adults is critical. If the spray is applied too early (e.g. August), the adults may not have become sufficiently active to be killed; if the spray is applied too late (e.g. mid-October), the adults may have already laid their eggs. Adhere to product label. See APVMA for current chemical options.
In New Zealand, a new and promising biopesticide based on the naturally occurring bacterium Yersinia entomophaga, is being evaluated in field trials in 2015-16.
Acknowledgements Top
This article was compiled by Sandra Hangartner, Garry McDonald (cesar) and Paul Umina (cesar).
References/Further Reading Top
Tasmanian Department of Primary Industries, Parks Water and Environment. 2010. African black beetle. Biosecurity fact sheet. http://dpipwe.tas.gov.au/Documents/africanblackbeetle.pdf
Bailey PT. 2007. Pests of field crops and pastures: identification and control. CSIRO Publishing. Melbourne. Australia.
Bulinski J and Matthiessen JN. 2002. Poor efficacy of the insecticide chlorpyrifos for the control of African black beetle (Heteronychus arator) in eucalypt plantations. Crop Protection 21: 621-627.
Bulinski J, Matthiessen JN and Alexander R. 2006. Development of a cost-effective, pesticide-free approach to managing African black beetle (Heteronychus arator) in Australian eucalyptus plantations. Crop Protection 25: 1161-1166.
Fisher D and Learmonth S. 2001. African black beetle in vineyards. Bulletin No. 4500, Agdex 241/622. Department of Agriculture and Food Western Australia. http://research.agwa.net.au/wp-content/uploads/2012/09/RT-99-8-African-Black-Beetle-in-Vineyards.pdf
Gerard PJ, Bell NL, Eden TM, King WM, Mapp NR, Pirie MR and Rennie GM. 2013. Black beetle persistence in Waikato and Bay of Plenty following the 2007-08 outbreak. Proceedings of the New Zealand Grassland Association 75, 235-240. http://www.grassland.org.nz/viewpublication.php?pubID=376
Micic S and Learmonth S. 2015. Identifying soil beetle pests – African Black Beetle. Department of Agriculture and Food Western Australia. https://www.agric.wa.gov.au/pest-insects/identifying-soil-beetle-pests?page=0%2C1
Matthiessen J and Learmonth S. 1991. African Black Beetle, Farmnote No. 17/91, Agdex 622, Agriculture Western Australia, Perth.
Nicholas P, Magarey P and Watchel M (eds). 1994. Diseases and Pests – Grape Production Series Number 1, Winetitles, Adelaide.
| Date | Version | Author(s) | Reviewed by |
|---|---|---|---|
| February 2015 | 1.0 | Paul Umina (cesar), Sandra Hangartner and Garry McDonald (cesar) | Bill Kimber (SARDI) |
| March 2016 | 1.1 | Garry McDonald (cesar) | Bill Kimber (SARDI) |
What are PestNotes?
PestNotes are information sheets developed through a collaboration between Cesar Australia and the South Australian Research and Development Institute (SARDI). Copyright: © All material published in PestNotes is copyright protected by Cesar Australia and SARDI and may not be reproduced in any form without written permission from both agencies.
Disclaimer
The material provided in PestNotes is based on the best available information at the time of publishing. No person should act on the basis of the contents of this publication without first obtaining independent, professional advice. PestNotes may identify products by proprietary or trade names to help readers identify particular products. We do not endorse or recommend the products of any manufacturer referred to. Other products may perform as well as or better than those specifically referred to. Cesar Australia and PIRSA will not be liable for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information in this publication. Any research with unregistered pesticides or products referred to in PestNotes does not constitute a recommendation for that particular use.

