Bluegreen aphid
Acyrthosiphon kondoi

Photo by Andrew Weeks, Cesar Australia
Summary Top
Bluegreen aphids are widespread in Australia. They are most common in spring but are also active in autumn and winter. Heavy infestations can cause damage to plants by direct removal of nutrients, deforming leaves and causing plants to wilt and become yellow. Bluegreen aphids are grey-green to blue-green coloured, attacking lupins, lucerne, medics and clovers. They are distinguished from other aphids by their long legs, antennae and cornicles.
Occurrence Top
Bluegreen aphids are widely distributed and found in all states of Australia. They are most common in spring but are also active in autumn and winter. Occurrences of damaging numbers in pasture legumes are irregular.
Description Top
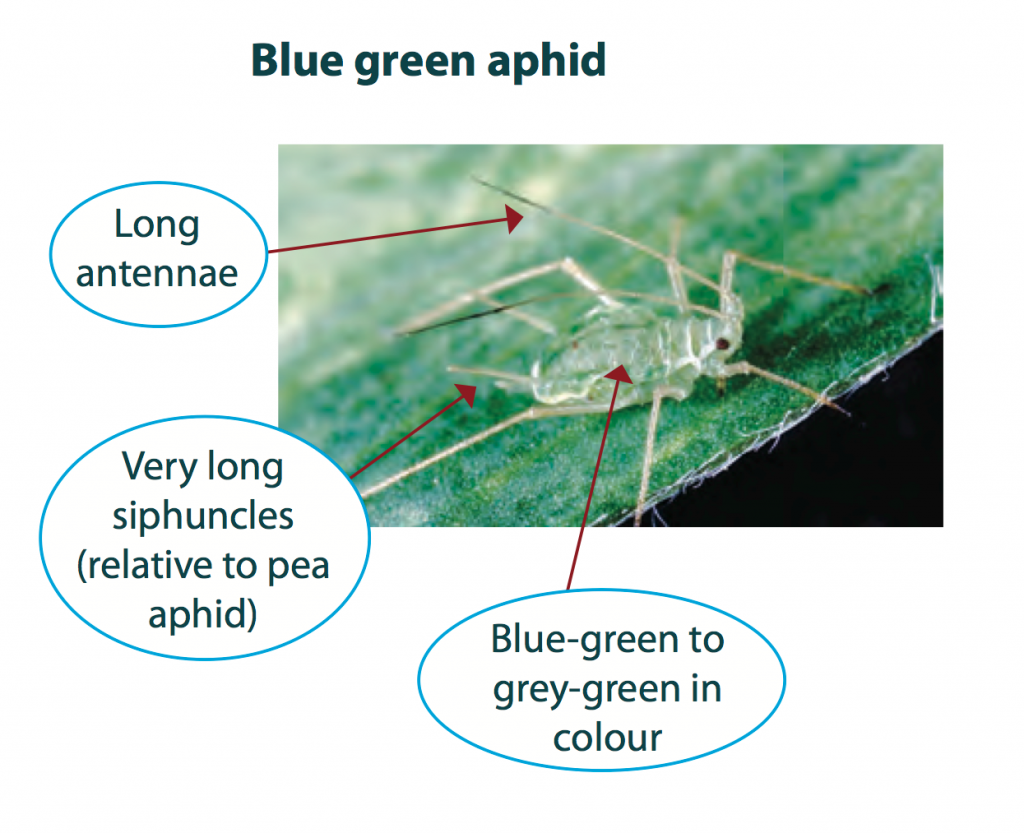
Aphids are a group of soft-bodied bugs commonly found in a wide range of crops and pastures. Identification of crop aphids is very important when making control decisions. Distinguishing between aphids can sometimes be challenging. It can be easier in the non-winged form but is more difficult with winged aphids.
Adults grow up to 3 mm long, are oval shaped, with long legs and antennae. They have two large cornicles that extend beyond the base of the abdomen. Both the winged and wingless forms are a matte bluish-green colour. Nymphs are similar to adults but are smaller in size.


Lifecycle Top
Winged aphids fly into crops from broad leaf weeds, pasture legumes and medics, and colonies of aphids start to build up within the crop. Aphids can reproduce both asexually and sexually, however in Australia, the sexual phase is often lost. Aphids reproduce asexually whereby females give birth to live young, which are often referred to as clones.
Nymphs, which do not have wings, go through several growth stages, moulting at each stage into a larger individual. Mature females can produce many generations during the growing season. Development rates are dependent on temperature and either cooler, or extremely hot temperatures slow development.
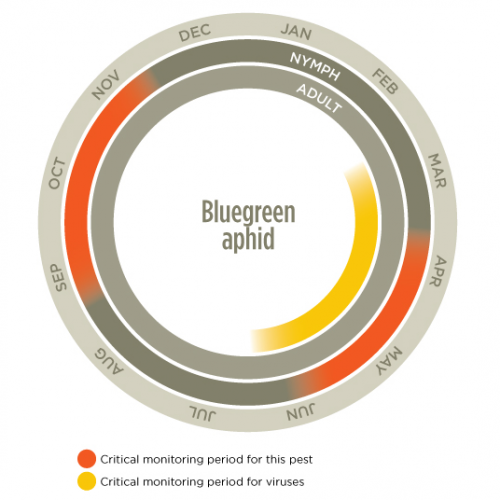
Behaviour Top
Bluegreen aphids are active during autumn and winter but are most prominent during spring. Bluegreen aphids feed on the upper leaves, stems and terminal buds of host plants. They are normally found on the upper part of the plant, particularly on the growing points.

Similar to Top
Other aphids, in particular the green peach aphid and pea aphid.
Crops attacked Top
Lucerne, annual medics and subterranean clover pastures, lupins, vetch, chickpeas and faba beans.
Damage Top
Direct feeding damage:
Bluegreen aphids cause feeding damage to upper leaves, stems and terminal buds of host plants. Heavy infestations can cause damage to plants by direct removal of nutrients, deforming leaves and causing plants to wilt and become yellow. In lucerne and medics, heavy infestations cause stunted growth, leaf curling and leaf drop. Dry matter production can be reduced. Secretion of honeydew can cause secondary fungal growth, which inhibits photosynthesis and can decrease plant growth.
In general, aphids have the greatest impact on crops when soil moisture is limited, and in these instances yields can be reduced. In 2010 there were reports of a new, more virulent bluegreen biotype that caused damage to some varieties of lentils, annual medic and sub-clover that were previously thought to be tolerant to bluegreen aphids.
Indirect damage (virus transmission):
Bluegreen aphids cause indirect damage by spreading plant viruses. Aphids spread viruses between plants by feeding and probing as they move between plants and paddocks. Bluegreen aphids transmit important plant viruses including cucumber mosaic virus (CMV) and bean yellow mosaic virus (BYMV).
Bluegreen aphids transmit important plant viruses including cucumber mosaic virus and bean yellow mosaic virus
Monitor Top
Regularly monitor vulnerable crops during bud formation to late flowering. Aphids will generally move into paddocks from roadsides and damage will first appear on crop edges. Aphid distribution may be patchy, so monitoring should include at least 5 sampling points over the paddock. Inspect at least 20 plants at each sampling point. Visually search for aphids looking at the youngest inflorescence of each plant. Look for clusters of aphids or symptoms of leaf-curling.
Aphid infestations can be reduced by heavy rain events or sustained frosts. If heavy rain occurs after a decision to spray has been made, but before the insecticide has been applied, check the crop again to determine if treatment is still required.
Economic thresholds Top
Canola:
• NSW: >50% of plants with clusters 25 mm long on stem or 4-5 stems per m2 with cluster 50 mm long on stems (Hertel et al. 2013).
• WA: 20% of plants infested (Berlandier et al. 2010) OR >10% of plants with >25 mm of stem infested (Berlandier & Baker 2007).
Lupins:
• NSW: Treat at first indication of virus infected plants, or first appearance of aphid clusters on flowering spikes (Hertel et al. 2013)
• WA: >30% of inflorescences infested with 30 or more aphids, however, also take into account the aphid tolerance of the variety (Berlandier 1999)
Faba beans: (Vic) – 10% of plants infested.
Lucerne, Chickpeas & Lentils: No thresholds available
Management options Top
Biological
There are many effective natural enemies of aphids. Hoverfly larvae, lacewings, ladybird beetles and damsel bugs are known predators that can suppress populations. Aphid parasitic wasps lay eggs inside bodies of aphids and evidence of parasitism is seen as bronze-coloured enlarged aphid ‘mummies’. As mummies develop at the latter stages of wasp development inside the aphid host, it is likely that many more aphids have been parasitized than indicated by the proportion of mummies. Naturally occurring aphid fungal diseases (Pandora neoaphidis and Conidiobolyus obscurus) can also affect aphid populations.

Cultural
Select cultivars that are less susceptible to aphid feeding damage. Sow crops early to enable plants to begin flowering before aphid numbers peak in spring. Control summer and autumn weeds in and around crops to reduce the availability of alternate hosts between growing seasons. Use a high sowing rate to achieve a dense crop canopy, which will assist in deterring aphid landings.
Chemical
There are several insecticides registered against bluegreen aphids in legume and pulse crops. Pirimicarb provides effective control and has little impact on beneficial insects compared with broad-spectrum chemicals. A border spray in autumn/early winter, when aphids begin to move into crops, may provide sufficient control without the need to spray the entire paddock. The use of insecticide seed treatments can delay aphid colonisation and reduce early infestation and aphid feeding.
Before making spray decisions, monitor for beneficial insects. Insecticide applications generally harm beneficials, many of which are more susceptible to broad-spectrum chemicals than pest insects.
Acknowledgements Top
This article was compiled by Paul Umina (cesar) and Bill Kimber (SARDI).
References/Further Reading Top
Bailey PT. 2007. Pests of field crops and pastures: Identification and Control. CSIRO Publishing, Melbourne, Australia.
Bellati J, Mangano P, Umina P and Henry K. 2012. I SPY. Insects of Southern Australian Broadacre Farming Systems Identification Manual and Education Resource. Department of Primary Industries and Resources South Australia (PIRSA), the Department of Agriculture and Food Western Australia (DAFWA) and cesar Pty Ltd.
Berlandier FA. 1999. Aphids in lupin crops: their biology and control. Agriculture Western Australia Farmnote No. 44/99.
Berlandier FA and Baker GJ. 2007. Winter oilseeds. In: Pests of field crops and pastures: identification and control. (ed. PT Bailey) pp 135-154. CSIRO Publishing: Melbourne.
Berlandier FA, Severtson D and Mangano P. 2010. Aphid management in canola. Farmnote 440. DAFWA. Perth.
Edwards OR, Franzmann B, Thackray D and Micic S. 2008. Insecticide resistance and implications for future aphid management in Australian grains and pastures: a review. Australian Journal of Experimental Agriculture 48: 1523-1530.
GRDC. 2010. Aphids and viruses in pulse crops fact sheet. http://www.grdc.com.au/uploads/documents/GRDC_FS_AphidsAndViruses.pdf
Hertel K, Roberts K and Bowden P. 2013. Insect and mite control in field crops. New South Wales DPI. ISSN 1441-1773.
Humphries A, Peck D, Robinson S, Oldach K, Glatz R and Howie J. 2010. A new highly virulent bluegreen aphid causes severe damage in previously tolerant pasture and grain legumes. Proceedings of the 15th Agronomy Conference 2010. Lincoln, New Zealand.
Lawrence L. 2009. Taking the flight to aphids. Farming Ahead 215: 49 -51.
Moran N. 1992. The evolution of aphid life cycles. Annual Review of Entomology 37: 321-348.
Valenzuela I and Hoffmann AA. 2015. Effects of aphid feeding and associated virus injury on grain crops in Australia. Austral Entomology. DOI: 10.1111/aen.12122
| Date | Version | Author(s) | Reviewed by |
|---|---|---|---|
| March 2015 | 1.0 | Paul Umina (cesar) and Bill Kimber (SARDI) | Paul Umina (cesar) and Alana Govender (cesar) |
What are PestNotes?
PestNotes are information sheets developed through a collaboration between Cesar Australia and the South Australian Research and Development Institute (SARDI). Copyright: © All material published in PestNotes is copyright protected by Cesar Australia and SARDI and may not be reproduced in any form without written permission from both agencies.
Disclaimer
The material provided in PestNotes is based on the best available information at the time of publishing. No person should act on the basis of the contents of this publication without first obtaining independent, professional advice. PestNotes may identify products by proprietary or trade names to help readers identify particular products. We do not endorse or recommend the products of any manufacturer referred to. Other products may perform as well as or better than those specifically referred to. Cesar Australia and PIRSA will not be liable for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information in this publication. Any research with unregistered pesticides or products referred to in PestNotes does not constitute a recommendation for that particular use.

