Cabbage aphid
Brevicoryne brassicae

Photo by Andrew Weeks, Cesar Australia
Summary Top
Cabbage aphids are one of several aphid species that can be problematic in canola crops. They are a widespread, introduced pest that forms distinctive colonies of many individuals on the flowering spikes of canola during late winter and spring. Cabbage aphids transmit a number of plant viruses, which can cause significant losses in crops.
Occurrence Top
Cabbage aphid are widely distributed and found in all states of Australia. They are very common as a pest of canola, with peak abundance typically during spring. Numbers of cabbage aphids in canola crops are on the rise in some regions as temperatures gradually become warmer.
Description Top
Aphids are a group of soft-bodied bugs commonly found in a wide range of crops and pastures. Identification of crop aphids is very important when making control decisions. Distinguishing between aphids can sometimes be challenging. It can be easier in the non-winged form but is more difficult with winged aphids.
Cabbage aphids grow up to 3 mm in length, have a dull grey-green coloured body and can be winged or wingless. Nymphs are similar to adults but are smaller in size and do not have wings. Cabbage aphid colonies have a characteristic blue-grey appearance and are normally covered in a thick, whitish powder.


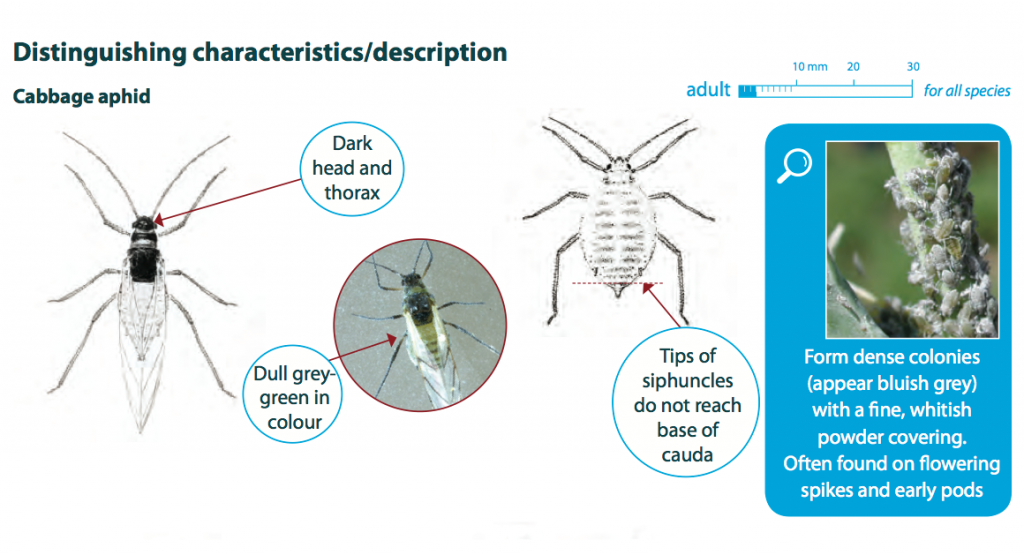
Lifecycle Top
Winged aphids fly into crops from mustard, wild radish, wild turnip and other cruciferous plants, and colonies of aphids start to build up within the crop. Aphids can reproduce both asexually and sexually, however, in Australia, the sexual phase is often lost. Aphids reproduce asexually whereby females give birth to live young.
Temperatures during autumn and spring are optimal for aphid survival and reproduction. During these times, the aphid populations may undergo several generations. Populations peak in late winter and early spring; development rates are particularly favoured when daily maximum temperatures reach 20-25°C. Cabbage aphids are more tolerant to cold weather than the turnip aphid and will continue to develop slowly at temperatures around 5-9°C.
Young wingless aphid nymphs develop through several growth stages, moulting at each stage into a larger individual. Plants can become sticky with honey-dew excreted by the aphids. When plants become unsuitable or overcrowding occurs, the population produces winged aphids (alates), which can migrate to other plants or crops.
Behaviour Top
Cabbage aphids initially invade crops from the edges and are most commonly found on the under-surface of leaves. Host plants within 20 m of the crop edge are an extremely likely source of aphids, plants within 20-50 m are a likely source, and plants beyond 100 m are less likely to be a risk. Nonetheless, aphid flight distances will vary with prevailing winds.
As the crop advances aphids may form dense colonies on floral parts of plants, especially at the maturing, terminal flowering spike. This is occasionally occurs in association with the turnip aphid.
Similar to Top
Other aphids, in particular the turnip aphid and green peach aphid.
Crops attacked Top
Predominantly canola, but also other cruciferous forage crops. Alternative weed hosts include mustard, wild radish and wild turnip.
Damage Top
Direct feeding damage:
Infestations start when winged aphids fly into crops from autumn weeds. Cabbage aphid infestations occur most frequently in canola from early flowering to late pod development.
Adults and nymphs suck sap from plants and high numbers can result in yield loss by reducing pod set, pod fill and grain quality. Colonies often become evident by the distortion and discoloration (yellowing) of infested parts. Canola is particularly susceptible to aphid damage during bud formation through to late flowering.
Secretion of honeydew by aphids can cause secondary fungal growth, which inhibits photosynthesis and can decrease plant growth.
Indirect damage (virus transmission):
Cabbage aphids cause indirect damage by spreading plant viruses. Aphids spread viruses between plants by feeding and probing as they move between plants and paddocks. The ability to transmit particular viruses differs with each aphid species and viruses may be transmitted in a persistent or non-persistent manner (see below). This influences the likelihood of plant infection.
Viruses affecting canola
Cabbage aphids are important vectors of plant diseases including beet western yellows virus (BWYV, syn. turnip yellows virus), cauliflower mosaic virus (CaMV) and turnip mosaic virus (TuMV) all of which cause damage in canola. These viruses are widespread and surveys have found that in many situations most crops have some infected plants. These viruses are not seed-borne. They survive in weeds or volunteer host plants during the summer and are then spread from these plants into crops by aphids that act as a vector for transmission. Yield loss is greater in crops that have been infected as seedlings. Viral infection can occur past the rosette stage of canola growth but these often have little effect on yield.
BWYV is termed a persistent virus and infects the phloem of plants. Persistent viruses are carried in the aphid’s body and can be transmitted to healthy plants during feeding; the aphid remains infective throughout their life. CaMV and TuMV are non-persistent viruses and are only retained in the aphid mouthparts for less than 4 hours.
Viral infection can occur past the rosette stage of canola growth but these often have little effect on yield.
Monitor Top
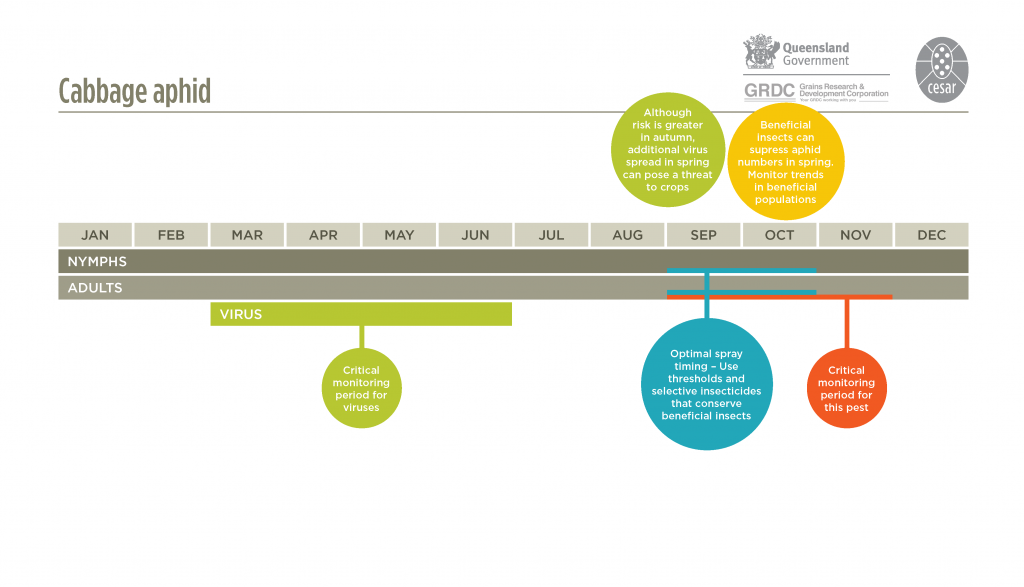
Cabbage aphids are most likely to be detected early on the undersurface of leaves in the bottom portion of the crop canopy and on plants within the first 10–20 m of crop edge or other non-crop areas, such as weedy patches within or adjacent to canola fields. Crop damage will first appear on crop edges.
Monitor for cabbage aphids weekly from late winter onwards, but especially during flowering and grain fill. Cabbage aphids will generally move into paddocks from roadsides and damage will first appear on crop edges. Cabbage aphids are usually found on the terminal flowering spikes. Aphid distribution may be patchy, so monitoring should include at least five sampling points over the paddock. Inspect at least 20 plants at each sampling point. For disease-prone areas, regular aphid monitoring from autumn onwards is recommended to detect aphids moving into crops, particularly along paddock edges.
Symptoms of virus infections are very variable, from no visual indication, to stunted red plants and stiffening of leaves for BWYV (syn. turnip yellows virus), chlorotic ring spots and mottling for CaMV and yellow mosaic patterning and tip necrosis for TuMV. Autumn is the critical infection period; the earliest-sown crops usually have the highest infection incidence.
Aphid infestations can be reduced by heavy rain events or sustained frosts. If heavy rain occurs after a decision to spray has been made, but before the insecticide has been applied, check the crop again to determine if treatment is still required.
Economic thresholds Top
http://ipmguidelinesforgrains.com.au/pests/aphids/
Canola:
- NSW: > 50% of plants with clusters 25 mm long on stem or 4-5 stems per m2 with clusters 50 mm long on stems (Hertel et al. 2013).
- WA: 20% of plants infested (Berlandier et al. 2010) or >10% of plants with > 25 mm of stem infested (Berlandier & Baker 2007).
When determining economic thresholds for aphids, it is critical to consider several other factors before making a decision. Most importantly, the current growing conditions and moisture availability should be assessed. Crops that are not moisture stressed have a greater ability to compensate for aphid damage and will generally be able to tolerate far higher infestations than moisture stressed plants before a yield loss occurs.
Thresholds for managing aphids to prevent the incursion of aphid-vectored virus have not been established and will be much lower than any threshold to prevent yield loss via direct feeding.
Management options Top
Biological
There are many effective natural enemies of aphids. Hoverfly larvae, lacewings, ladybird beetles and damsel bugs are known predators that can suppress populations. Aphid parasitic wasps lay eggs inside bodies of aphids and evidence of parasitism is seen as bronze-coloured enlarged aphid ‘mummies’. As mummies develop at the latter stages of wasp development inside the aphid host, it is likely that many more aphids have been parasitized than indicated by the proportion of mummies. Naturally occurring aphid fungal diseases (Pandora neoaphidis and Conidiobolyus obscurus) can also suppress aphid populations.
If the parasitism trend increases over time, there are good prospects that aphid populations will be controlled naturally.
Cultural
Control summer and autumn weeds in and around crops, particularly wild radish and wild turnip, to reduce the availability of alternate hosts between growing seasons. Ensure final herbicide application to control weeds is at least 10-14 days before sowing and Brassica weeds are controlled within at least 20 m of the crop. Where feasible, sow into standing stubble and use a high sowing rate to achieve a dense crop canopy, which will assist in deterring aphid landings. Sow at recommended times; earlier sown crops usually have a greater incidence of viral infection.
Chemical
The use of insecticide seed treatments can delay aphid colonisation and reduce early infestation, aphid feeding and the spread of viruses.A border spray in autumn/early winter, when aphids begin to move into crops, may provide sufficient control without the need to spray the entire paddock. Pirimicarb is registered against cabbage aphids. Pirimicarb has little impact on beneficial insects compared with broad-spectrum chemicals. Consider adding a wetting agent to the spray mix to help the insecticide penetrate the aphid’s waxy surface. See APVMA for current chemical options. Avoid the use of broad-spectrum ‘insurance’ sprays and apply insecticides only after monitoring and distinguishing between aphid species. Consider the populations of beneficial insects before making a decision to spray, particularly in spring when these natural enemies can play a very important role in suppressing aphid populations if left untouched.
Rotating chemical groups and taking advantage of biological control are essential to extend the useful life of the available chemistries.
Acknowledgements Top
This article was compiled by Paul Umina (cesar) and Sandra Hangartner.
References/Further Reading Top
Bailey PT. 2007. Pests of field crops and pastures: Identification and Control. CSIRO Publishing, Melbourne, Australia.
Bellati J, Mangano P, Umina P and Henry K. 2012. I SPY Insects of Southern Australian Broadacre Farming Systems Identification Manual and Education Resource. Department of Primary Industries and Resources South Australia (PIRSA), the Department of Agriculture and Food Western Australia (DAFWA) and cesar Pty Ltd.
Berlandier FA and Baker GJ. 2007. Winter oilseeds. In: Pests of field crops and pastures: identification and control. (ed. PT Bailey) pp 135-154. CSIRO Publishing: Melbourne.
Berlandier FA, Severtson D and Mangano P. 2010. Aphid management in canola. Farmnote 440. DAFWA. Perth.
Blackman RL and Eastop VF. 2000. Aphids on the world’s crops: an identification and information guide. John Wiley and Sons, England.
Coutts BA and Jones, RAC. 2000. Viruses infecting canola (Brassica napus) in south-west Australia: incidence, distribution, spread and infection reservoir in wild radish (Raphanus raphinistrum). Australian Journal of Agricultural Research 51: 925–936.
Coutts BA, Hawkes JR and Jones RAC. 2006. Occurrence of Beet western yellows virus and its aphid vectors in over-summering broad-leafed weeds and volunteer crop plants in the grainbelt region of south-western Australia. Australian Journal of Agricultural Research 57: 975–982.
Day MF and Irzykiewicz. 1953. Feeding Behaviour of the Aphids Myzus Persicae and Brevicoryne Brassicae, Studied With Radiophosphorus. Australian Journal of Biological Sciences 6: 98-108.
Edwards OR, Franzmann B, Thackray D, Micic S. 2008. Insecticide resistance and implications for future aphid management in Australia grains and pastures: a review. Australian Journal of Experimental Agriculture 48: 1523-1530.
Gu H, Fitt GP and Baker GH. 2007. Invertebrate pests of canola and their management in Australia: a review. Australian Journal of Entomology 46: 231-243.
Hertel K, Roberts K and Bowden P. 2013. Insect and mite control in field crops. New South Wales DPI. ISSN 1441-1773.
Hughes RD. 1963. Population dynamics of the cabbage aphid, Brevicoryne brassicae (L.). The Journal of Animal Ecology 32: 393-424.
Jones, R and Hawkes, J. 2002. Yield losses caused when Beet western yellows virus infects canola. Agribusiness Crop Updates. Department of Agriculture, Western Australia.
Jones R, Coutts B, Smith L and Hawkes J. 2003. Benefits provided by treating canola seed with imidacloprid seed dressing. Agribusiness Crop Updates. Department of Agriculture, Western Australia.
King C, Jacob HS and Berlandier F. 2006. The influence of water deficiency on the relationship between canola (Brassica napus L.), and two aphid species (Hemiptera: Aphididae), Lipaphis erysimi (Kaltenbach) and Brevicoryne brassicae (L.). Australian Journal of Agricultural Research 57: 439-445.
Marcroft S, Potter T and Jones R. 2011. Canola diseases: The back pocket guide. GRDC.
Miles PW, Aspinall D and Rosenberg L. 1982. Performance of the cabbage aphid, Brevicoryne brassicae (L.), on water-stressed rape plants, in relation to changes in their chemical composition. Australian Journal of Zoology 30: 337-346.
Moran N. 1992. The evolution of aphid life cycles. Annual Review of Entomology 37: 321-348.
Parry HR, Macfadyen S and Kriticos DJ. 2012. The geographical distribution of Yellow dwarf viruses and their aphid vectors in Australian grasslands and wheat. Australasian Plant Pathology Society 41: 375-387.
Price, L (Northern Growers Alliance). 2010. Cereal Aphids Fact Sheet (Northern Region). GRDC.
Schwinghamer M and Schilg M. 2003. The virus situation in chickpeas, faba beans and canola. Proceedings GRDC Update – Dubbo.
Schwinghamer M, Schilg MA, Walsh JA, Bambach RW, Cossu RM, Bambridge JM, Hind-Lanoiselet TL, McCorkell BE and Cross P. 2014. Turnip mosaic virus: potential for crop losses in the grain belt of New South Wales, Australia. Australasian Plant Pathology 43: 663-678.
Severtson D, Flower K and Nansen C. 2015. Nonrandom Distribution of Cabbage Aphids (Hemiptera: Aphididae) in Dryland Canola (Brassicales: Brassicaceae). Environmental entomology 44: 767-779.
Valenzuela I and Hoffmann AA. 2014. Effects of aphid feeding and associated virus injury on grain crops in Australia. Austral Entomology. DOI: 10.1111/aen.12122
Wratten, K. 2002. Viruses in canola in NSW. Proceedings GRDC Update – Wagga Wagga.
| Date | Version | Author(s) | Reviewed by |
|---|---|---|---|
| March 2015 | 1.0 | Paul Umina (cesar) and Sandra Hangartner | Alana Govender (cesar) and Bill Kimber (SARDI) |
| March 2016 | 1.1 | Garry McDonald (cesar) | Bill Kimber (SARDI) |
What are PestNotes?
PestNotes are information sheets developed through a collaboration between Cesar Australia and the South Australian Research and Development Institute (SARDI). Copyright: © All material published in PestNotes is copyright protected by Cesar Australia and SARDI and may not be reproduced in any form without written permission from both agencies.
Disclaimer
The material provided in PestNotes is based on the best available information at the time of publishing. No person should act on the basis of the contents of this publication without first obtaining independent, professional advice. PestNotes may identify products by proprietary or trade names to help readers identify particular products. We do not endorse or recommend the products of any manufacturer referred to. Other products may perform as well as or better than those specifically referred to. Cesar Australia and PIRSA will not be liable for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information in this publication. Any research with unregistered pesticides or products referred to in PestNotes does not constitute a recommendation for that particular use.

