Diamondback moth
Plutella xylostella
Other common names
Cabbage moth, DBM

Photo by Andrew Weeks, Cesar Australia
Summary Top
Diamondback moths are 10 mm long and grey-brown in colour with a white uneven stripe down the centre of their back. Larvae are small yellow-green caterpillars and are a major pest of canola and other brassica crops. There are significant issues with insecticide resistance. Biological control by small parasitic wasps (Diadegma semiclausum, Apanteles ippeus and Diadromus collaris) is most important.
Insecticide resistance in diamondback moth is a significant issue.
Occurrence Top
Diamondback moths are distributed across Australia, but are most common in southern states. Although they can be found throughout the year, they are most abundant in canola crops during spring.
Description Top
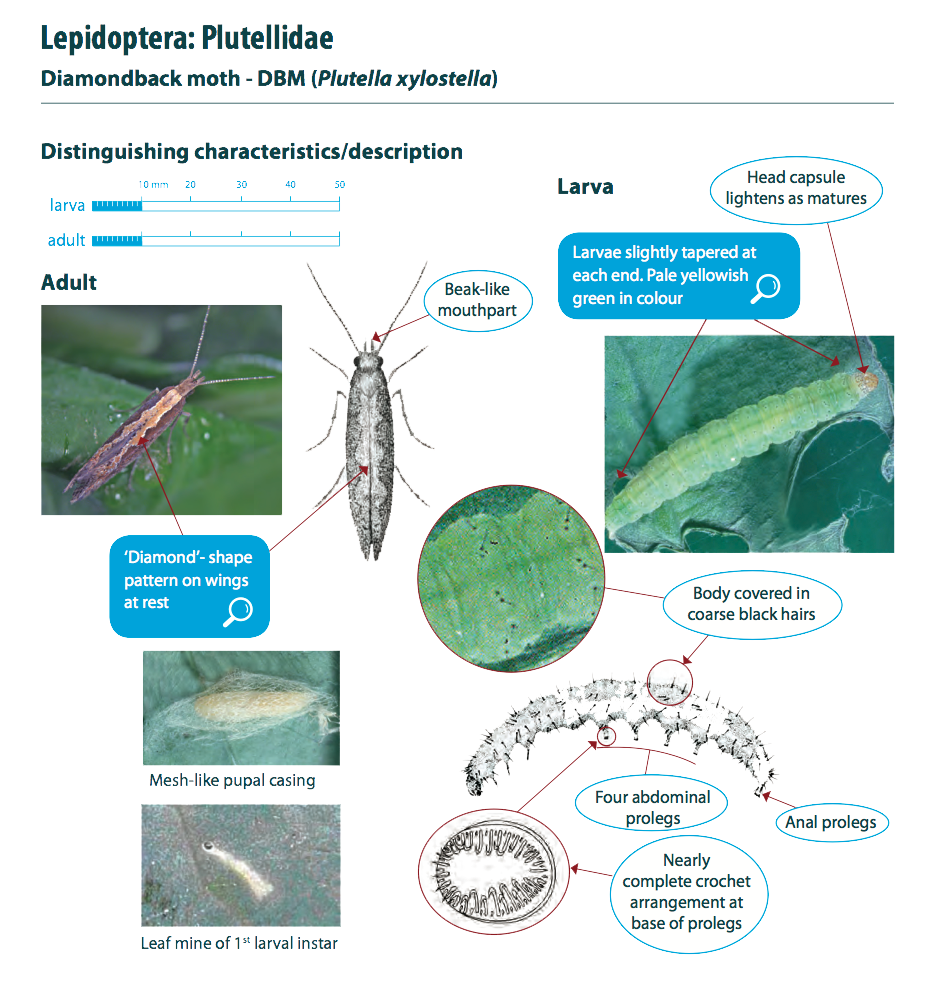
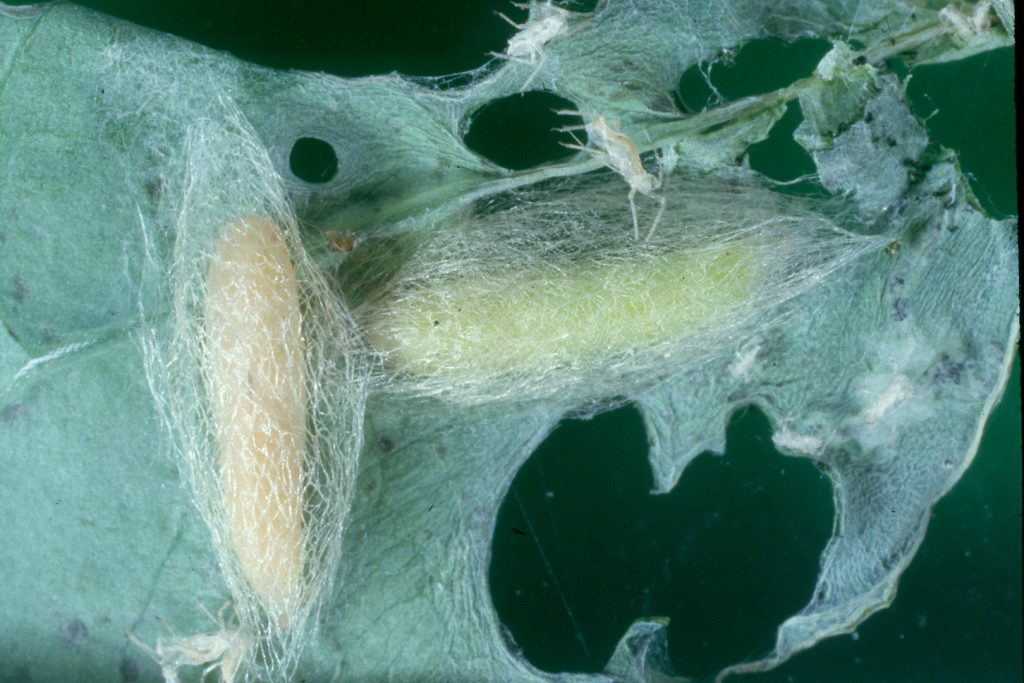
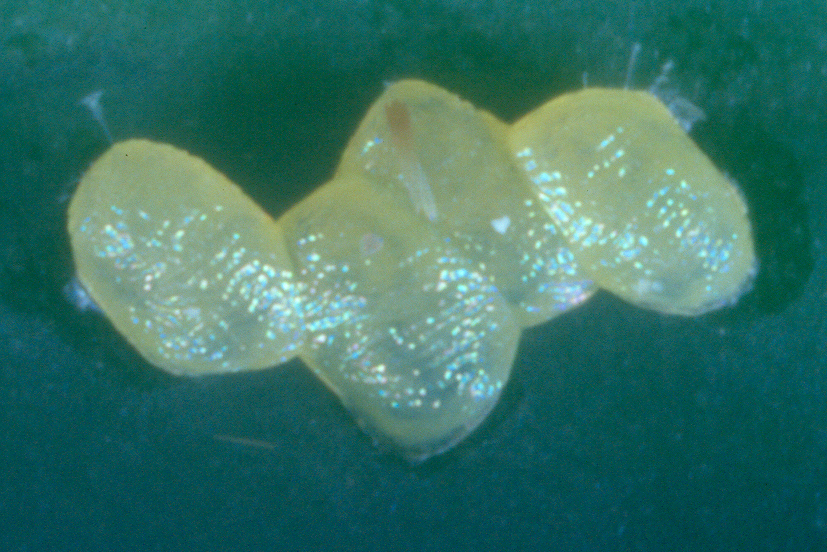
Diamondback moths, also referred to as DBM or cabbage moths, are 10 mm long and grey-brown in colour with a white uneven stripe down the centre of their back. When the moths are at rest this uneven shape forms a ‘diamond’ pattern on their wings. They have beak-like mouthparts. Eggs are pale yellow, oval and about 0.5 mm long. Larvae are pale yellowish-green in colour, can grow up to 10mm in length and have a body shape that is slightly tapered at each end. The 2nd and 3rd instar larvae have a dark head. Pupae are a cream-green colour, which darkens before the adult moth emerges.


Lifecycle Top
Female DBM lay more than 100 eggs in their lifetime, either singularly or in clusters along leaf veins, shortly after mating. Eggs hatch in about 4-6 days. 1st instars tunnel into leaf tissue. Larvae go through four instar stages before they pupate. The rate of development from eggs to moths depends on temperature; faster in warm weather and slower in cool weather. At 28°C the lifecycle takes 14 days, whereas at 12°C the lifecycle takes more than 100 days.
Behaviour Top
Adults are active at dusk and during the night, and usually do not move far within a crop. They can migrate long distances on winds. First instars are not visible as they live and feed inside leaf tissue. 2nd and 3rd instars feed on the leaf surface, usually on the undersides, or on developing flowers, and wriggle vigorously when disturbed.
Summer rainfall stimulates the growth of a green bridge for DBM survival, especially wild radish, Lincoln weed and volunteer canola.
Similar to Top
Small larvae may be confused with cabbage centre grub, vegetable looper, native budworm and cabbage white butterfly caterpillars. They also superficially resemble beneficial hover fly larvae.
Crops attacked Top
Canola and other brassica crops. Alternative hosts include wild radish and Lincoln weed.
Damage Top
Small larvae feed on leaves, producing ‘windows’ and small holes in the leaves. Feeding is often concentrated on the underside of leaves. Older larvae graze on stems and pods. As flowering progresses, increasing numbers of larvae move to the floral buds, flowers and pods. Larvae may feed on small young pods while mature pods are usually only surface grazed. Heavy infestations can cause severe defoliation, which can reduce crop yields. Canola can tolerate considerable foliar feeding damage without impacting yield.

Monitor Top
Canola should be monitored from late winter to late spring/early summer. Search for the presence of larvae on leaves, buds and flowers, especially during flowering and podding. Sample crops by sweep netting. Take a minimum of 5 sets of 10 sweeps in several parts of the crop and calculate the average number of the larvae per 10 sweeps. If no diamondback moth are detected the crop should be monitored again in a fortnight.
It is important to re-monitor 5-7 days after a spray treatment.
Economic thresholds Top
Canola:
• Australia wide: >10 larvae per 10 sweeps for pre-early flowering, >50 larvae per 10 sweeps for early-mid flowering through to pod formation, and >100 larvae per 10 sweeps for late flowering through to podding (GRDC 2010).
Management options Top
Biological
Small parasitic wasps (Diadegma semiclausum, Apanteles ippeus and Diadromus collaris) are most important. Other generalist predators include brown lacewings, spiders and damsel bugs. Naturally occurring fungal diseases may be significant under certain conditions (rainfall, high humidity & warm temperatures). The disease Zoophthora radicans can cause >90% mortality in DBM populations.
DBM pupae parasitised by Diadegma semiclausum are distinguishable visually from unparasitised healthy pupae.
Weather conditions can dramatically affect populations. Greater than 5-8 mm of rain in 24 hours can reduce larvae numbers as they are dislodged and drowned or killed by disease.


Cultural
Control summer and autumn weeds in and around paddocks to reduce the survival of larval populations.
Chemical
There are several insecticides registered. Diamondback moths can evolve insecticide resistance readily and some populations are difficult to control with insecticides. No single insecticide application will completely eliminate the population. In years when populations are large, a two-spray strategy is recommended. Bacillus thuringiensis (Bt) is an effective insecticide on diamondback moth and is a ‘soft’ chemical on natural enemies. Apply Bt late in the day or early evening to minimize UV breakdown, and ensure the insecticide is applied within 2 hours of mixing.
When using agricultural chemicals always read the label or permit. Only use products registered for the targeted crop. Do not exceed the maximum crop rate. Adhere to the withholding period.
Acknowledgements Top
This article was compiled by Greg Baker and Bill Kimber (SARDI).
References/Further Reading Top
Bailey PT. 2007. Pests of field crops and pastures: Identification and Control. CSIRO Publishing, Melbourne, Australia.
Baker G. 2001. Monitoring canola crops for cabbage moth. PIRSA Fact Sheet 144/620.
Dosdall LM, Soroka JJ, Olfert O. 2011. The Diamondback moth in canola and mustard: Current pest status and future prospects. Prairie Soils & Crops Journal 4: 66-76.
Henry K and Baker G. 2008. Diamondback moth in canola. FS/03/08. South Australian Research and Development Institute, Primary Industries and Resources South Australia.
GRDC. 2010. Diamondback moth a sporadic but serious pest. Western and Southern Regions Factsheet.
Walden K, Mangano P, Baker G and Umina P. 2010. Diamond back moth in canola. GRDC FactSheet. Coretext.
| Date | Version | Author(s) | Reviewed by |
|---|---|---|---|
| January 2015 | 1.0 | Bill Kimber and Greg Baker (SARDI) | Garry McDonald (cesar) |
What are PestNotes?
PestNotes are information sheets developed through a collaboration between Cesar Australia and the South Australian Research and Development Institute (SARDI). Copyright: © All material published in PestNotes is copyright protected by Cesar Australia and SARDI and may not be reproduced in any form without written permission from both agencies.
Disclaimer
The material provided in PestNotes is based on the best available information at the time of publishing. No person should act on the basis of the contents of this publication without first obtaining independent, professional advice. PestNotes may identify products by proprietary or trade names to help readers identify particular products. We do not endorse or recommend the products of any manufacturer referred to. Other products may perform as well as or better than those specifically referred to. Cesar Australia and PIRSA will not be liable for any loss, damage, cost or expense incurred or arising by reason of any person using or relying on the information in this publication. Any research with unregistered pesticides or products referred to in PestNotes does not constitute a recommendation for that particular use.

